SARS-Cov-2 turned out to become the first pandemic of the millennium. It has become more and more clear with the virus’s growth how little we know, as a society, about dealing with a large scale disease such as Covid-19. This virus has been able to magnify critical issues in our healthcare. Among these the difficulty of correctly and rapidly diagnosing the disease certainly represents one of the most relevant.
At the moment the rhino-faringeal swab is the only effective test we have, but laboratories can’t manage to satisfy the medical structures’ growing demand for testing, due to the rapid expansion of the virus, in a reasonable amount of time. Infact the rhino-faringeal swab, in addition to being extremely invasive and counting on reagents not always available, requires up to 24 hours to produce a verdict on the patient’s positivity.
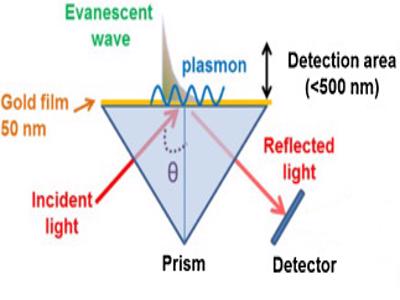
We propose a diagnosing sensor which drastically cuts these waiting times. The solution we will be working on is a re adaptation from an immunologic sensor based on the superficial plasmon resonance technique (aka SPR).
By exploiting the high ACE2 protein’s affinity with the SARS-Cov-2, our idea is to fix this protein on the sensor’s gold film by binding it to a thiolic group.
The strengths of such a device would be high reliability on reproducible results, low cost, reusability and, above all, low response time, which could be reduced up to only 15 minutes.
Medical tags
- Clinical need
- Point-of-care diagnosis
- Area
- Cellular pathology
- Technology
- In vitro diagnostic device
- Project keywords
- COVID, DesignCompetition2020, Plasmons, gold, spr, covid-19, diagnostic, optic, bradichinine, ace-2, biosensor, SARS-cov-2, SARS
- Device classification
- IIa